Lithiumfluorid
LiF
Lithiumfluorid (LiF) besitzt die kleinste Transmissionskante im VUV-Bereich. Diese liegt bei 0,116 µm. Hier werden bereits 50 % der auftreffenden Strahlung transmittiert. Im Wellenlängenbereich von etwa 0,2‐6 µm, liegt der Transmissionsanteil bei über 90 %. Das Material besitzt einen geringen Brechungsindex, wodurch eine Antireflexbeschichtung in den Optiken nicht notwendig ist. Bei Temperaturen von über 400 °C wird Lithiumfluorid durch Luftfeuchtigkeit abgebaut, eine Verwendung in trockener Umgebung ist jedoch bis 600 °C möglich. Das Material ist empfindlich gegen thermischen Schock. Lithiumfluorid wird mittels Czochralski- oder Bridgman-Methode gezogen.
Lithiumfluorid ist dank seiner Transmissionseigenschaften das bevorzugte Material für die Herstellung von Optiken für den VUV-Bereich. Weitere Verwendung findet das Material als passiver Güteschalter für aktive Laserelemente oder als Röntgenmonochromator. Für Anwendungen im Röntgenbereich ist es notwendig, dass das Lithiumfluorid keine Störungen im Kristallgitter aufweist.
Optische Eigenschaften | |
|---|---|
Transmissionsbereich in µm (Minimum 10%) | 0,12‐8,7 |
Transmissionsbereich in µm (Minimum 50%) | 0,12‐7,4 |
Brechungsindex @633nm | 1,39126
|
Reflexionsverluste in % an 1 Oberfläche | 2,65 @633nm
|
Reflexionsverluste in % an 2 Oberflächen | 5,2 @600nm
|
dN/dT in 1/K | -16 · 10-6 @1,15µm
|
Physikalische Eigenschaften | |
|---|---|
Dichte in g/cm3 | 2,639 |
Schmelzpunkt in °C | 848 |
Spezifische Wärmekapazität in J/(kg · K) | 1562
|
Thermische Leitfähigkeit in W/(m · K) | 11,3
|
Thermische Ausdehnung in 1/K | 37 · 10-6
|
Dielektische Konstante | 9
|
Wasserlöslichkeit in g/100g | 0,27 @18°C |
Mohs-Härte | 4
|
Knoop-Härte in kg/mm² | 102
|
Materialtyp | Einkristall, synthetisch
|
Kristalltyp | kubisch, flächenzentriert, NaCl-Struktur |
Kristallstruktur | Fm3m
|
Gitterkonstante in Å | a = 4,02
|
Elastizitätskonstanten in GPa | C11 = 112 C12 = 45,6 C42 = 63,2 |
Elastizitätsmodul (E) in GPa | 64,79
|
Schubmodul (G) in GPa | 33,7 |
Kompressionsmodul (K) in GPa | 62,03 |
Biegefestigkeit in MPa | 10,9
|
Dehnungsgrenze in MPa | 11,2
|
Poissonzahl | 0,22 |
Spektrale Eigenschaften |
|---|
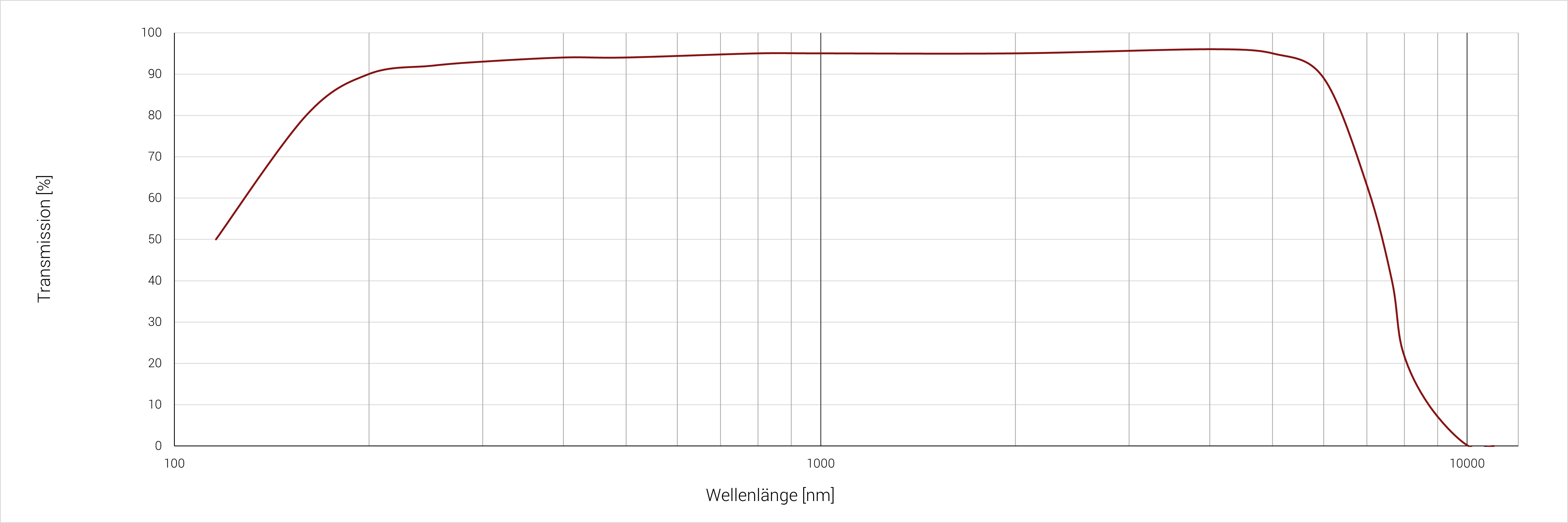 |
Probendicke: 2 mm |
| Optische Eigenschaften | |
|---|---|
| Transmissionsbereich in µm @10% Min. | 0,12 - 8,7 |
| Transmissionsbereich in µm @50% Min. | 0,12 - 7,4 |
| Brechungsindex @ 633nm | 1,39126 |
| Reflexionsverluste in % an 1 Oberfläche | 2,65 @ 633nm |
| Reflexionsverluste in % an 2 Oberflächen | 5,2 @ 600nm |
| dn/dT in 1/K | -16 · 10-6 @ 1,15µm |
| Physikalische Eigenschaften | |
|---|---|
| Dichte in g/cm³ | 2,639 |
| Schmelzpunkt in °C | 848 |
| Spezifische Wärmekapazität in J/(kg · K) | 1562 |
| Thermische Leitfähigkeit in W/(m · K) | 11,3 |
| Thermische Ausdehnung in 1/K | 37 · 10-6 |
| Dielektische Konstante | 9 |
| Wasserlöslichkeit in g/100g | 0,27 @ 18°C |
| Mohs-Härte | 4 |
| Knoop-Härte in kg/mm² | 102 |
| Materialtyp | Einkristall, synthetisch |
| Kristalltyp | kubisch flächenzentriert, NaCl-Struktur |
| Kristallstruktur | cF12 |
| Gitterkonstante in Å | a = 4,02 |
| Elastizitätskonstanten in GPa | C11 = 112 C12 = 45,6 C44 = 63,2 |
| Elastizitätsmodul (E) in GPa | 64,79 |
| Schubmodul (G) in GPa | 55,14 |
| Kompressionsmodul (K) in GPa | 62,03 |
| Biegefestigkeit in MPa | 10,9 |
| Dehnungsgrenze in MPa | 11,2 |
| Poissonzahl | 0,22 |
| Spektrale Eigenschaften | |
|---|---|
 |
|

