Calcit
Material
Calcite (CaCO3) is one of the most stable polymorphic calcium carbonates. On the Mohs hardness scale, this material defines hardness 3, i.e. it can be scratched with a copper coin. It dissolves in slightly acidic solutions. Water andCO2 from the environment are sufficient for easy decomposition. Transparent calcite is interesting because of its strong birefringent properties.
In optics, pure calcite in particular is used for polarizers (e.g. as a Glan-Foucault prism) or as a wave plate (λ/n plate) due to its strong birefringence.
Properties
Spectral properties
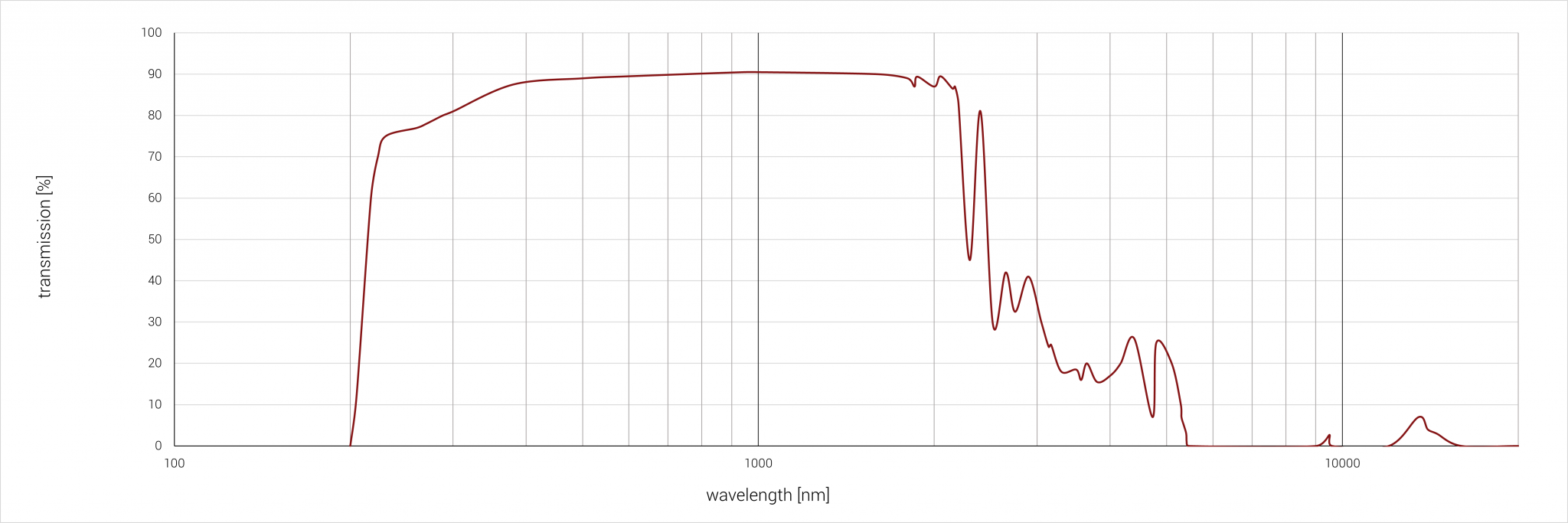
Sample thickness: 2 mm

